As the next-generation photovoltaic technology, organic solar cells have attracted extensive attention from academia and industry in the past two to three decades. Recently, oligomerized fused-ring electron acceptors (OFREAs) have emerged as small molecules/polymers due to their unique advantages (well-defined structure, good batch-to-batch reproducibility, good film-forming properties, low diffusion coefficient, and excellent stability, etc.) A strong competitor for bioacceptors, showing great potential to realize high-efficiency and high-stability organic photovoltaic devices. In the early stage, the team successfully built a high-efficiency (binary device efficiency 18.26%, ternary Organic solar cells with high device efficiency (18.73%), high stability (T80% = 2681 h) and low energy loss (0.493 eV) (CCS Chem., DOI: 10.31635/ccschem.31023.202202575).
Recently, the research group was invited to write a review on oligomerized fused-ring electron acceptors on Angew. Conformation and stacking, long-term stability, etc., and future prospects and challenges.
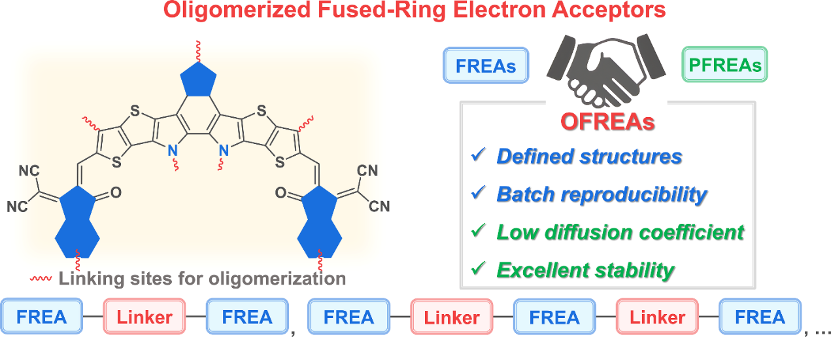
Figure 1. Potential oligomerization attachment sites and advantages of oligomerization fused-ring electron acceptors
1. History of oligomerized fused-ring electron acceptors
In early nonfullerene acceptors, oligomerization strategies have been widely used, such as perylenediimide oligomers with twisted structures. In recent years, small molecule fused ring electron acceptors and their polymeric derivatives have been developed rapidly. However, small-molecule materials often exhibit a tendency to diffuse rapidly, leading to morphological aging under thermal stress; polymeric acceptor materials suffer from poor batch-to-batch reproducibility due to molecular weight polydispersity. Based on the above problems, Professor Zou Yingping of Central South University proposed a new type of acceptor material called "Quasi-Polymer" for the first time in a 2020 opinion article (Chem, 2020, 6, 2147-2161). can be seen as the origin of the concept of oligomerized fused-ring electron acceptors.
2. Structural diversity
At present, the oligomerization fused ring electron acceptor is based on the Y-series electron acceptor as a monomer, because there are multiple potential oligomerization connection sites on this unit, such as the terminal unit, the N position on the pyrrole ring, A series of oligomerized fused-ring electron acceptors with rich structures have been rapidly developed based on the shoulder side chains and the central fused-ring core position, and the photoelectric conversion efficiency of organic photovoltaic devices based on them has also rapidly exceeded 18%. According to the current development of this kind of acceptor material, it can be divided into directly linked oligomers/homo-oligomers, rigidly linked oligomers, flexibly linked oligomers and direct condensed oligomers. Fused oligomers.
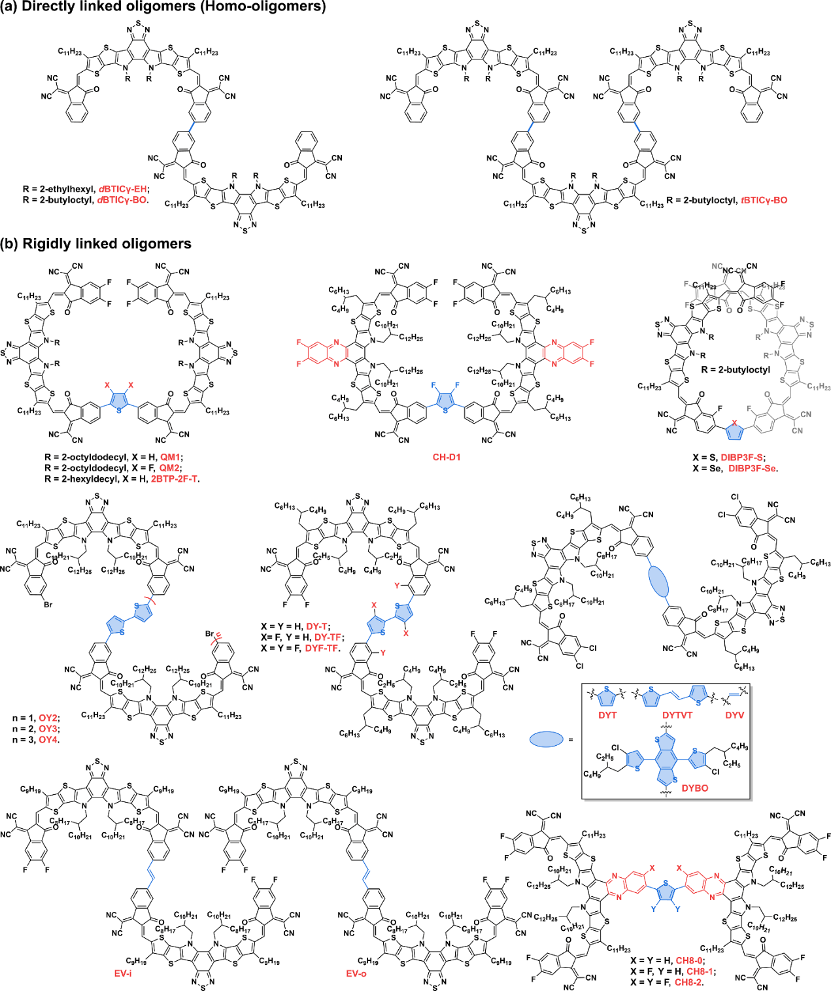
Figure 2. Chemical structures of direct-linked and rigid unit-linked oligomers
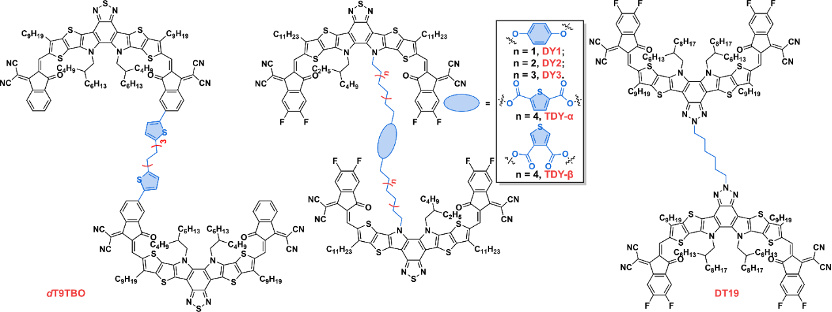
Figure 3. Chemical structures of flexible unit-linked oligomers
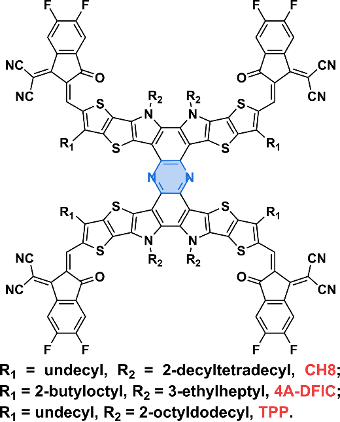
Figure 4. Chemical structures of directly fused oligomers
3. Synthesis method
Most dimerized fused-ring electron acceptors are currently prepared by palladium-catalyzed Stille coupling of asymmetric monobromomonomers and bistin-functionalized linking units (Scheme I). Among them, the asymmetric monobromine monomer is synthesized from a dialdehyde intermediate and two different terminal groups through a one-pot Knoevenagel condensation reaction. However, this one-pot method faces problems such as difficult separation and low yield. Recently, Professor Zhang Zhiguo from Beijing University of Chemical Technology proposed a facile synthetic method to prepare dimerized fused-ring electron acceptors (Route II). A2-Linker-A2 can be synthesized by Suzuki/Stille coupling reaction of diboronate or bistin-substituted linking unit with brominated end group (A2-Br). When A2-Linker-A2 is not soluble, it needs to be converted to a silane enol ether derivative to ensure high solubility. Finally, the nearly quantitative Lewis acid-catalyzed Knoevenagel condensation of A2-Linker-A2 (or its silylated product) and the monoaldehyde intermediate A1-DA′D-CHO can be rapidly prepared as acceptor materials. This approach provides a promising alternative for the modular synthesis of dimerized fused-ring electron acceptors.
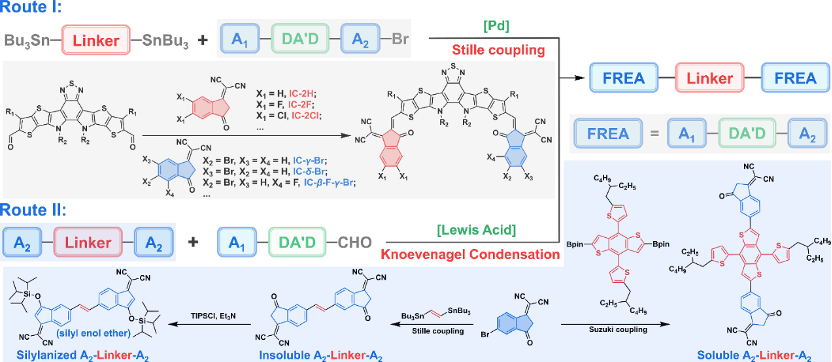
Figure 5. Typical synthesis of most dimerized fused-ring electron acceptors
4. Molecular conformation and packing behavior
The introduction of additional single bonds in the oligomerization of fused-ring electron acceptors will inevitably affect the molecular conformation of such acceptor materials, including possible conformational isomers and molecular planarity and rigidity, which in turn will affect the solid state. The molecular packing behavior exerts a significant influence and further affects the film morphology, charge transport properties and photovoltaic performance. For the directly connected oligomerized fused ring electron acceptor, due to the existence of its steric hindrance, there is a dihedral angle of about 35° between two adjacent monomers, while using thiophene/thiophene-ethylene-thiophene ( TVT), the planarity of oligomerized fused-ring electron acceptors is significantly improved. It is worth noting that the strategy of intramolecular non-covalent "conformational locks" (NoCLs) can effectively and rationally regulate the molecular conformation, and the introduction of non-covalent "conformational locks" can significantly enhance the planarity, rigidity and intermolecular π of the conjugated backbone. -π interaction, reducing recombination energy and energy loss, and then constructing efficient organic solar cells.
5. Long-term stability
Achieving long-term stability of organic solar cells is considered a key challenge for their future commercialization. In organic photovoltaic devices based on small-molecule acceptors, the main cause of thermal degradation is the aging of film morphology caused by molecular diffusion and self-aggregation, i.e., high diffusion coefficient leads to excessive aggregation under thermal stress. According to the relevant theory of polymer physics, for a given polymer, the longer the polymer chain, the higher the glass transition temperature. That is, the high molecular weight of the polymer tends to limit the movement of the molecular chains. Therefore, compared with small molecule acceptors, the oligomerized fused-ring electron acceptors have a higher glass transition temperature, which enables organic photovoltaic devices to exhibit thermally stable thin-film morphologies and long lifetimes. In terms of stability studies, the diffusion coefficient reflects the ability of oligomerized fused-ring electron acceptor molecules to move and thus affects the speed of morphology aging. In addition, characterization methods such as temperature/time-dependent atomic force microscopy and grazing incidence wide-angle X-ray scattering can reflect the morphology evolution and stability of oligomerized fused-ring electron acceptors. Finally, the T80% lifetime under light/thermal stress directly reflects the device stability based on oligomerized fused-ring electron acceptors.
6. Summary and Outlook
1) Studies have shown that even slight modifications to the linker units/sites can have a significant impact on the photovoltaic performance. Therefore, a more comprehensive study on the structure-property relationship of oligomerized fused-ring electron acceptors is urgently needed, and several directions for molecular engineering of oligomerized fused-ring electron acceptors are proposed: i) should start from Inspired by the molecular design of oligomeric fused-ring electron acceptors, rational use of central fused-ring core/side chain/end group engineering in the design of oligomerized fused-ring electron acceptors; ii) development of new rigid/flexible linking units for regulating molecular conformation and packing behavior to achieve favorable thin-film morphologies; iii) designing oligomerized fused-ring electron acceptors with star/multi-arm structures to enhance light-harvesting ability, improve charge-transport performance, and increase glass transition temperature.
2) The synthesis cost of materials should be considered. Currently, oligomerized fused-ring electron acceptors still face the problem of high production costs due to the complicated synthesis methods and low yields of fused-ring electron acceptor monomers. In recent years, a series of low-cost non-fused-ring electron acceptors (NFREAs) have been developed. The author proposes the concept of "oligomerized non-fused-ring electron acceptors (ONFREAs)", and oligomerized acceptors based on non-fused-ring electron acceptor units are expected to be the most efficient way to construct efficiency-stability-cost-balanced organic solar cell devices. good candidate.
3) Finally, the effects of thermal cycling and water/oxygen etc. on device stability should be investigated more deeply. In addition, large-area flexible devices/modules based on oligomerized fused-ring electron acceptors should be actively explored to further demonstrate their feasibility in future applications.
Gu Xiaobin, a doctoral candidate in the research group, is the first author of this article, and Associate Professor Zhang Xin and Professor Huang Hui are the co-corresponding authors. The author thanks the National Natural Science Foundation of China, Chinese Academy of Sciences and other related projects for funding.
Article details:Title:
Oligomerized Fused-Ring Electron Acceptors for Efficient and Stable Organic Solar Cells
Authors: Xiaobin Gu, Xin Zhang*, and Hui Huang*
Cite this by DOI: 10.1002/anie.202308496
Link: https://onlinelibrary.wiley.com/doi/10.1002/anie.202308496


