Organic solar cells (OSCs) have attracted widespread attention as a promising photoelectric conversion technology due to their flexibility, light weight, and printability. Thanks to the rapid development of non-fullerene acceptors (NFAs), the photoelectric conversion efficiency (PCE) of OSCs has exceeded 19%. The conjugated molecular skeleton of the receptor material can affect the geometric configuration, frontier orbital energy, aggregation behavior, etc., thereby determining device performance. So far, a large number of studies have focused on the design of the molecular skeleton of NFA, such as rigid fused ring cores and simple non-fused ring cores, corresponding to fused ring electron acceptors (FREAs) and non-fused ring electron acceptors (NFREAs) respectively. NFREAs show great potential in commercial applications due to their simple synthesis routes and low synthesis costs. As another important component of NFAs, alkyl side chains can effectively adjust the solubility, photophysical properties and stacking mode of molecules. However, the structure-activity relationship of the alkyl side chains of NFREAs on the molecular aggregation mode, especially the crystallization behavior, is still unclear, which limits the improvement of photovoltaic device performance.
Recently, our group designed and synthesized a series of new NFREAs: DPA-n (n = 3, 4, 5) by finely controlling the length of the alkyl side chain, and successfully controlled the crystal packing mode of the molecules. Although the three materials showed almost identical absorption spectra in solution, DPA-4 with a medium alkyl group length showed a higher extinction coefficient in the film state, indicating its tighter molecular packing in the film. In addition, single crystal XRD diffraction results show that multiple intermolecular C=O···H, S···F interactions can form extended interlocking planes, which promotes more efficient 3D electron transport and larger electron transport of DPA-4. The crystal packing coefficient is beneficial to improving the electron mobility of the material.
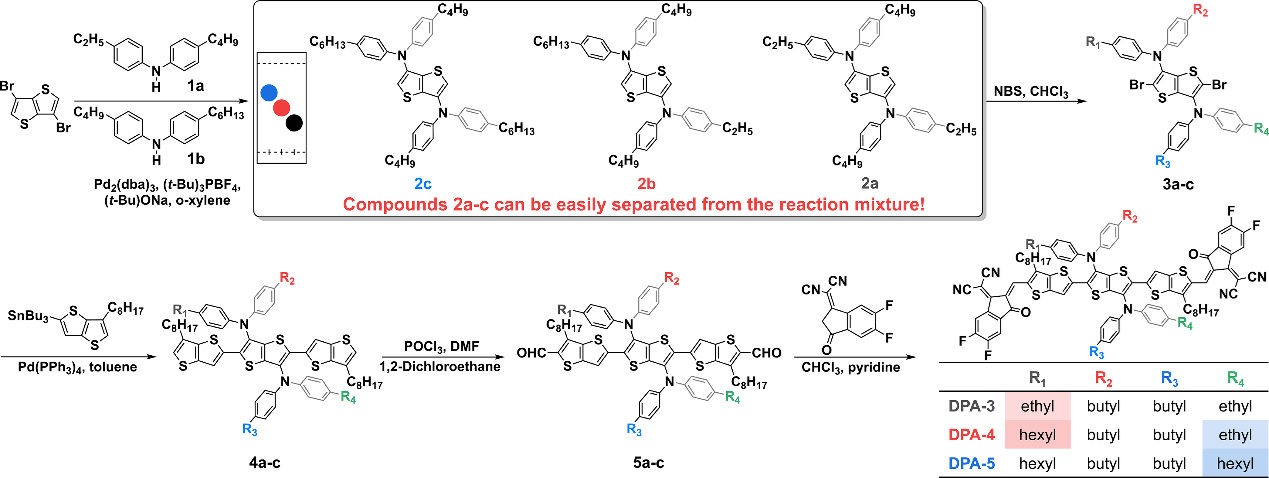
Figure 1. Molecular structure and synthetic route
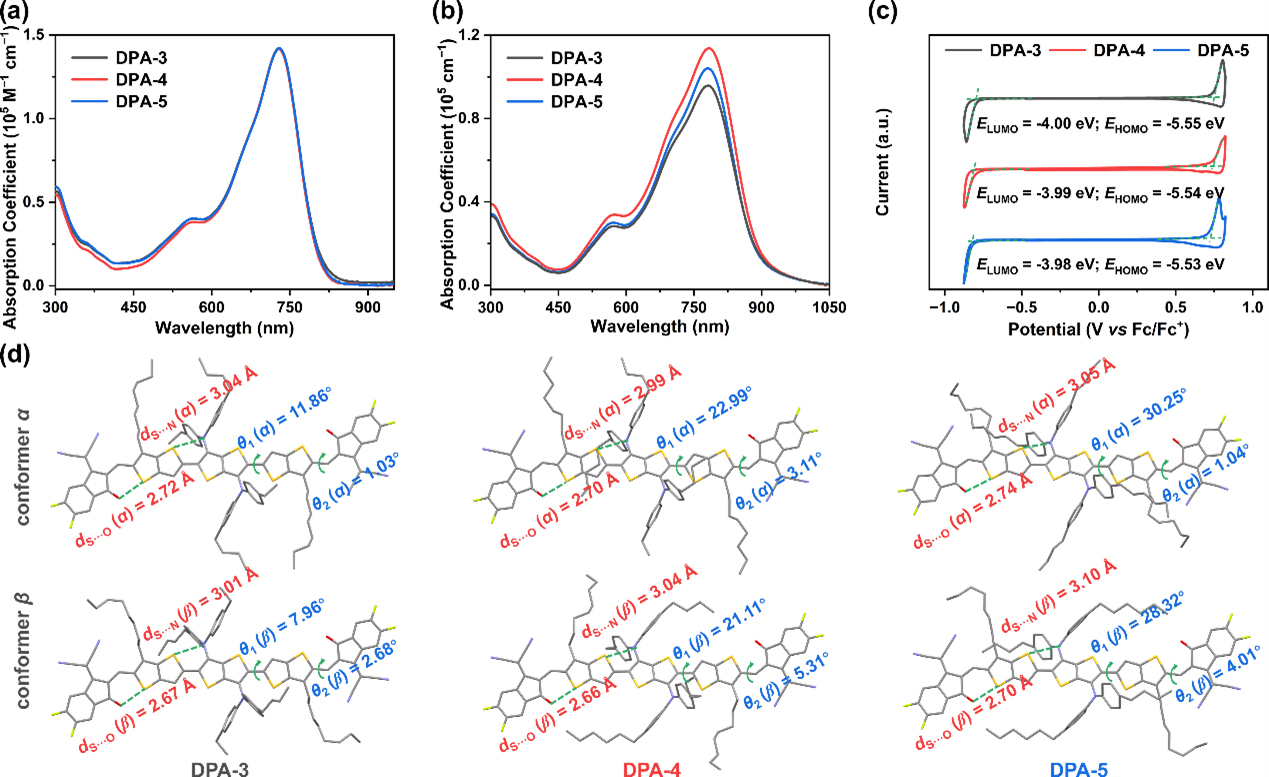
Figure 2. Absorption spectrum, energy level arrangement and molecular conformation in crystals
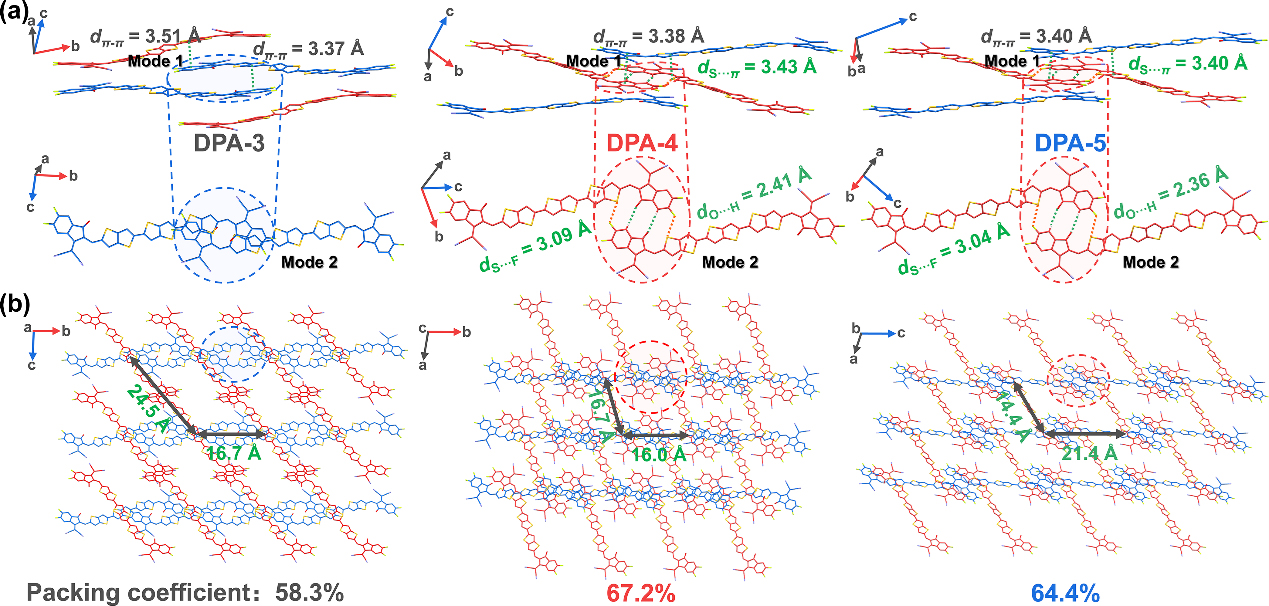
Figure 3. Crystal stacking pattern and three-dimensional crystal stacking network
Device physics studies show that when blended with the polymer donor D18 to prepare OSCs, DPA-4-based photovoltaic devices exhibit more efficient exciton dissociation and charge collection, higher and more balanced electron/hole mobility , lower energy loss, faster hole transfer and moderate phase separation, revealing the reasons for its higher EQE response and better device performance. The results show. The PCE of the device based on D18:DPA-4 is 16.67%, which is one of the highest values of devices based on NFREAs currently.
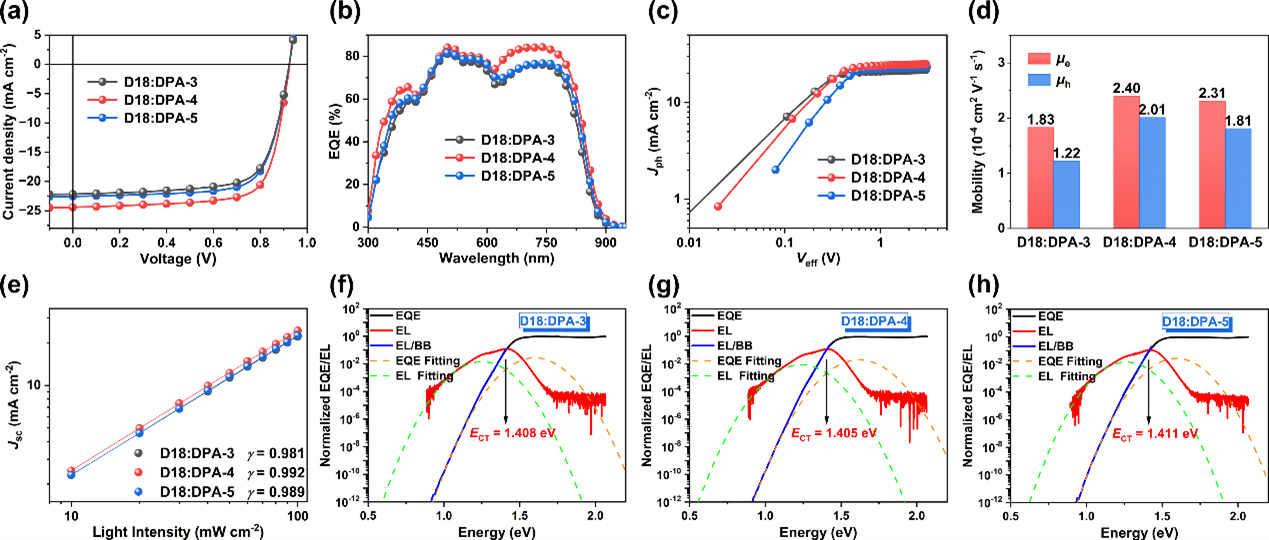
Figure 4. Photovoltaic performance and device physics study
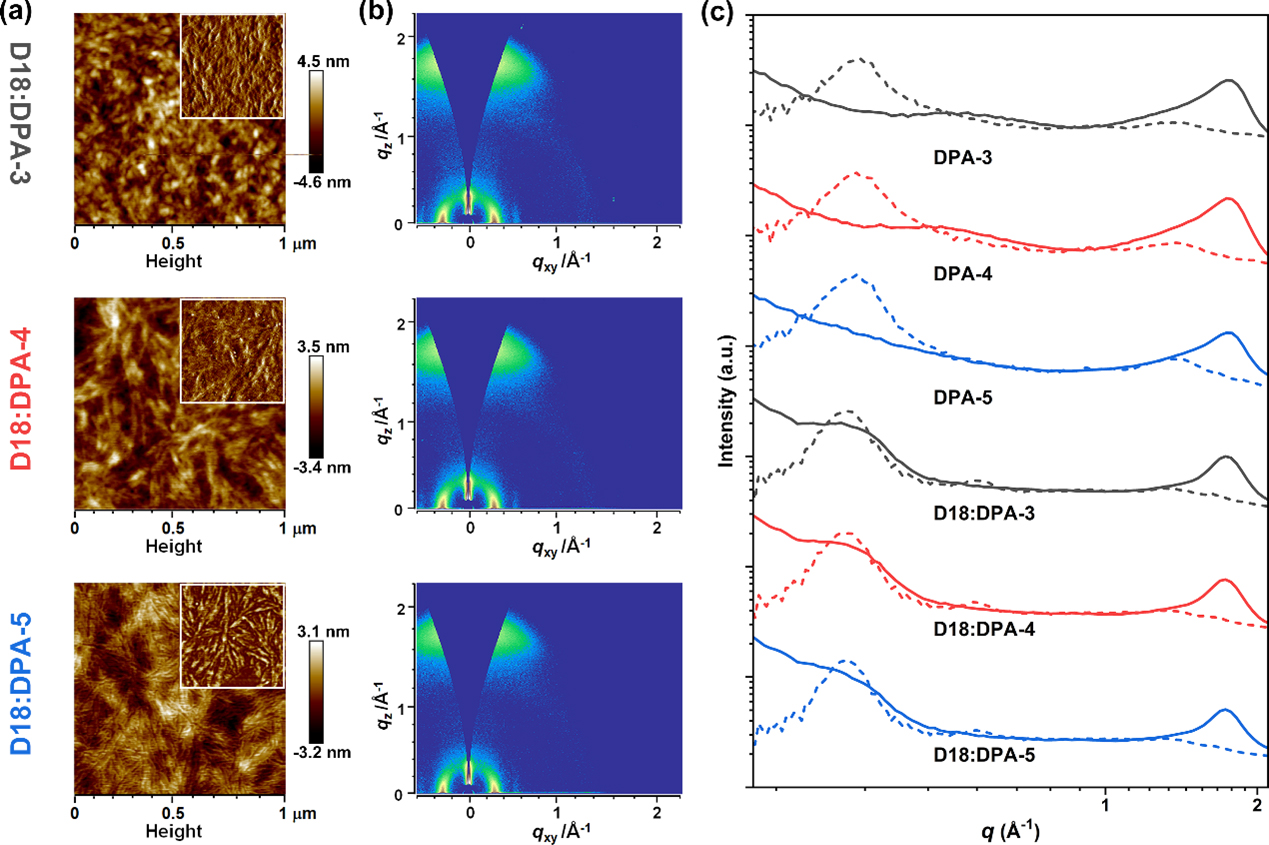
Figure 5. Film morphology study
This work successfully obtained a more efficient 3D crystal stacking network, optimized thin film stacking behavior and high-performance photovoltaic devices by finely adjusting the topology of the alkyl side chains of NFREAs, demonstrating the great development potential of NFREAs and laying the foundation for the design of high-performance NFREAs provide new thinking.
This work was published in "German Applied Chemistry" (Angew. Chem. Int. Ed. 2024, e202318143. https: //doi.org/10.1002/anie.202318143.) The first author of the paper is Han Ziyang, a doctoral candidate in the research group. The co-first authors are Dr. Zhang Cai'e and Dr. He Tengfei of Nankai University. The corresponding authors are Professor Huang Hui and Zhang Xin of the University of Chinese Academy of Sciences. Associate Professor.


