
First author: Zhang Xin, Gu Xiaobin
Corresponding author: Zhang Xin, Huang Hui; Corresponding unit: University of Chinese Academy of Sciences
Paper DOI:10.1021/acs.accounts.3c00813
This review focuses on summarizing the research group's work in designing low-cost, high-performance non-fused ring electron acceptors using non-covalent "conformational locking" strategies, and deeply discusses the molecular design principles and structure-property relationships of low-cost acceptors. , introduced the design of polynon-fused ring electron acceptors with a balance of efficiency-cost-stability, and finally pointed out the challenges and future development directions of non-fused ring electron acceptors.
【Background】
Organic solar cells have attracted widespread attention due to their light weight, flexibility, and translucency, and have gradually become one of the important candidates for renewable energy technologies. The photoactive layer composed of P-type electron donor and N-type electron acceptor materials is a key component of organic solar cell devices. The innovation and evolution of acceptor materials have greatly affected the development direction of this field. Since the advent of the first double-layer heterojunction structured organic solar cell in 1986, fullerene and its derivatives have dominated the development of receptors for nearly two decades due to their excellent isotropic charge transport properties, but the photoelectric properties can The disadvantage of limited tonality limits the efficiency of organic solar cells based on fullerene derivatives to around 12%. Until the breakthrough progress of non-fullerene acceptors, especially the invention of fused-ring electron acceptors, the field of organic solar cells has entered the fast lane of vigorous development. In recent years, thanks to the continuous innovation of molecular structures and the continuous optimization of device processing technology, the efficiency of organic solar cells based on fused ring electron acceptors has approached 20%. However, due to their complex chemical structures, fused-ring electron acceptors usually face lengthy synthesis routes, low yields, and high costs, which hinder the future large-scale industrialization of organic solar cells.
Non-fused ring electron acceptors have become strong candidates to replace fused ring electron acceptors due to their simple molecular structure, convenient synthetic route and low cost. From a chemical point of view, non-fused ring electron acceptors can be seen as tailoring the complex fused ring structure of the fused ring electron acceptor into a low fused ring degree or a simple aromatic ring structure, supplemented by intramolecular non-covalent interactions ( That is, non-covalent "conformational locks" (NoCLs) lock the planar conformation, thereby constructing a structurally simple but coplanar π-conjugated skeleton.
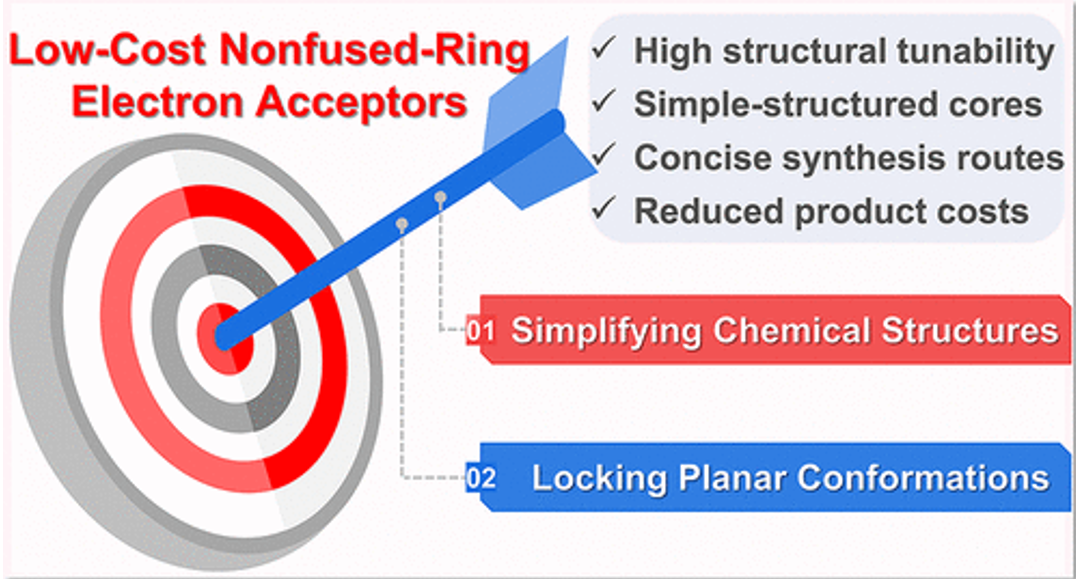
Figure 1. Design ideas and advantages of non-fused ring electron acceptors
【Quick overview of full text】
1. Simplify the molecular structure: from fused ring core to non-fused ring core
Non-fused ring electron acceptors are usually composed of low-densified binary/three-membered fused ring units or even simple aromatic (hetero)aromatic rings, and these basic units are connected to each other through carbon-carbon single bonds. However, the presence of additional single bonds inevitably leads to conformers or distorted molecular conformations, which significantly affects their optoelectronic properties and intermolecular packing behavior. To solve this problem, non-covalent "conformational lock" strategies are often introduced into the design of non-fused ring electron acceptors, thereby effectively limiting the rotation of carbon-carbon single bonds and locking planar conformations.
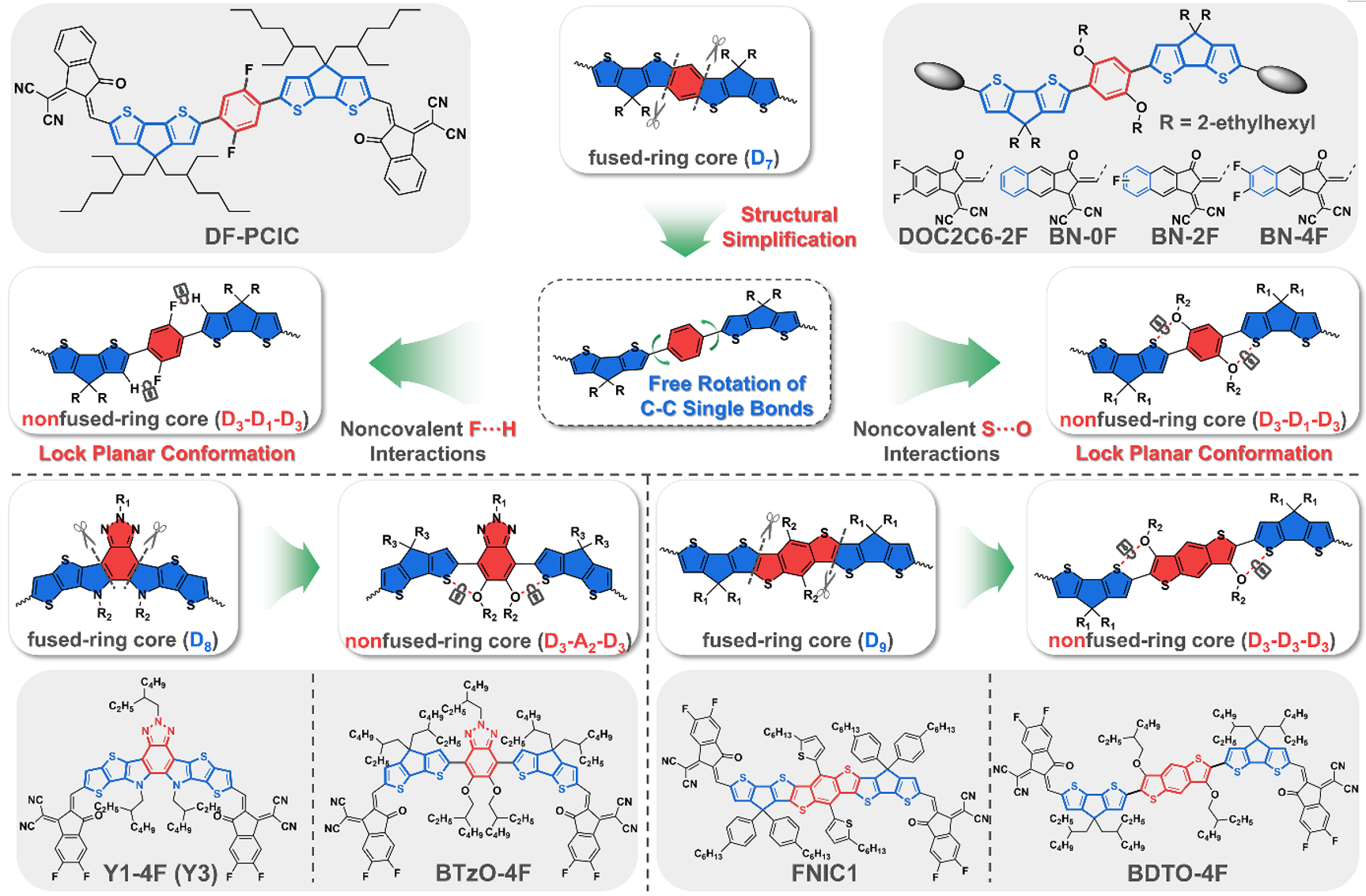
Figure 2. Schematic diagram of structural simplification and coplanarization of non-fused ring electron acceptors.
In order to describe the fused ring structure concisely, the author uses the symbol Dn (or An) to represent the electron-donating (or electron-withdrawing) unit, where n represents the number of fused rings. For example, the widely used cyclopentadithiophene unit can be represented as D3. The authors describe the origins of nonfused-ring electron acceptors and subsequent efforts in molecular design by researchers in the field. It focuses on the author's non-covalent "conformation" cut from seven-membered fused ring core (D7), eight-membered fused ring core (D8) and nine-membered fused ring core (D9) through fluorine‧‧‧hydrogen and sulfur‧‧‧oxygen. A series of non-fused ring electron acceptors that lock the planarity of the skeleton have repeatedly set new records for the photoelectric conversion efficiency of non-fused ring electron acceptor-based organic solar cells. This shows that the simplification of highly fused ring structures and the introduction of non-covalent "conformational locks" can not only simplify the synthesis route of electron acceptor materials, but also endow them with a highly coplanar conjugated skeleton and photoelectric conversion comparable to fused ring electron acceptors. efficiency, highlighting the commercial application potential of non-fused ring electron acceptors.
2. Constructing a completely non-fused ring electron acceptor
It is worth noting that the above-mentioned non-fused ring electron acceptors all contain cyclopentadithiophene units with a three-membered fused ring structure, which can be regarded as 2,2′-bithiophene building blocks connected by bridging carbon atoms. This rigid structure and the 4-substituted alkyl side chain endow the cyclopentadithienyl material with excellent charge transport properties and good solubility. However, the synthesis methods of cyclopentadithiophene are relatively complex and not environmentally friendly, usually involving multi-step reactions and toxic reagents (organolithium, hydrazine, etc.). Therefore, it is crucial to further simplify cyclopentadithiophene to develop structurally simpler and lower-cost fully non-fused ring electron acceptors.
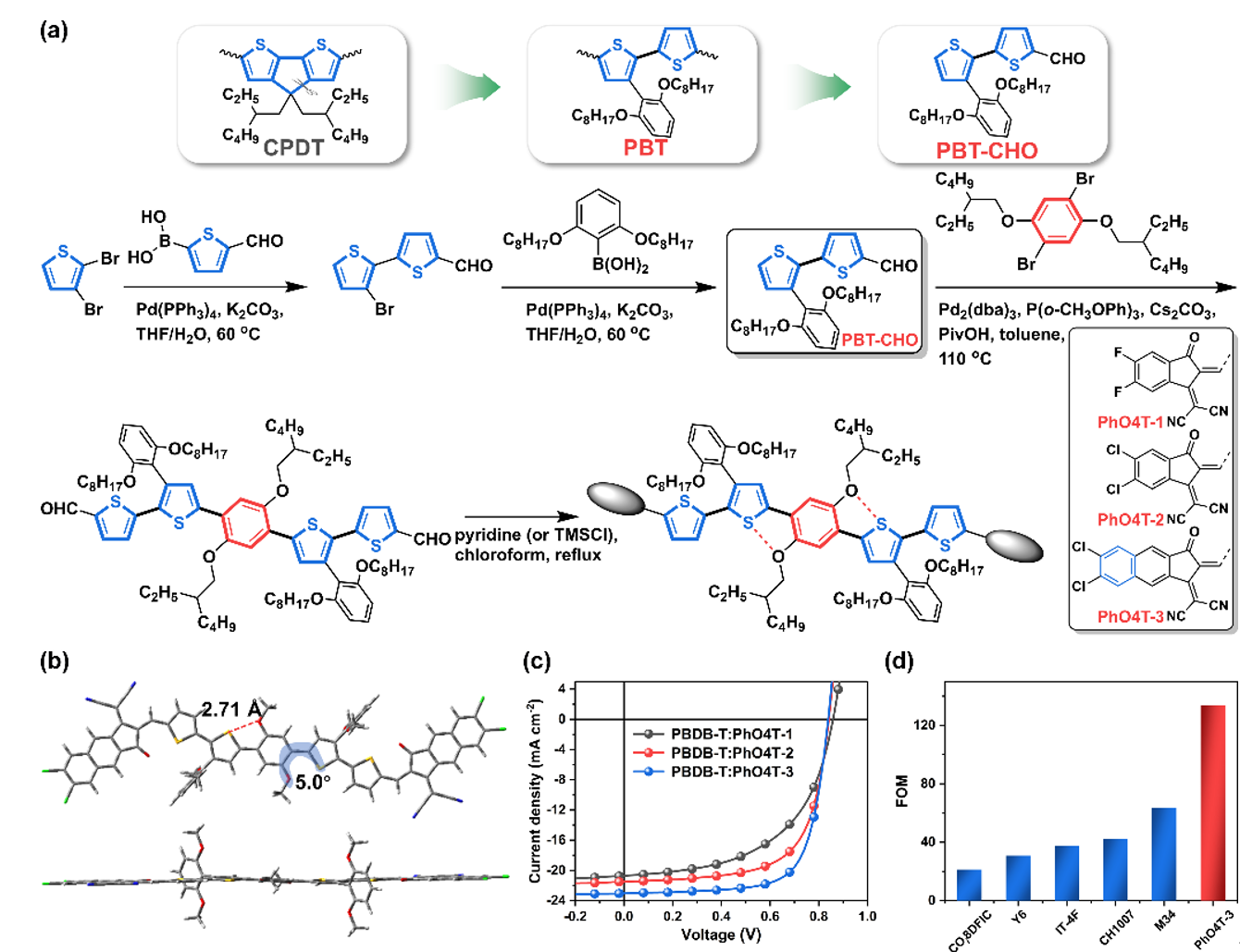
Figure 3. Simplification of cyclopentadithiophene unit and synthesis, characterization and quality factors of PhO4T series molecules
Based on the above considerations, the author used commercially available cheap 2,3-dibromothiophene as the starting material, and obtained a simple building block PBT based on 2,2'-bithiophene through a two-step green and non-toxic Suzuki cross-coupling reaction. Aldehyde product (PBT-CHO). Through further palladium-catalyzed direct arylation and Knoevenagel condensation reactions, the PhO4T series of completely non-fused ring electron acceptors can be obtained in high yields. Due to the presence of non-covalent "conformational locks" and sterically hindered side chains, this series of receptors exhibit a highly coplanar conjugated backbone and good solubility. Finally, the organic solar cell based on PhO4T-3 achieved a photoelectric conversion efficiency of 13.76% and a high figure of merit (FOM value), indicating that exploring non-fused ring electron acceptors with simpler structures is the key to achieving the efficiency-cost balance of organic solar cells. Promising approach.
3. Molecular engineering improves photovoltaic performance
Essentially, the structural, photoelectrochemical properties of the electron donor and acceptor units are critical for controlling molecular self-assembly, light harvesting capabilities, and photovoltaic performance. Therefore, molecular engineering of non-fused ring electron acceptor materials plays a key role in realizing high-performance organic solar cells.
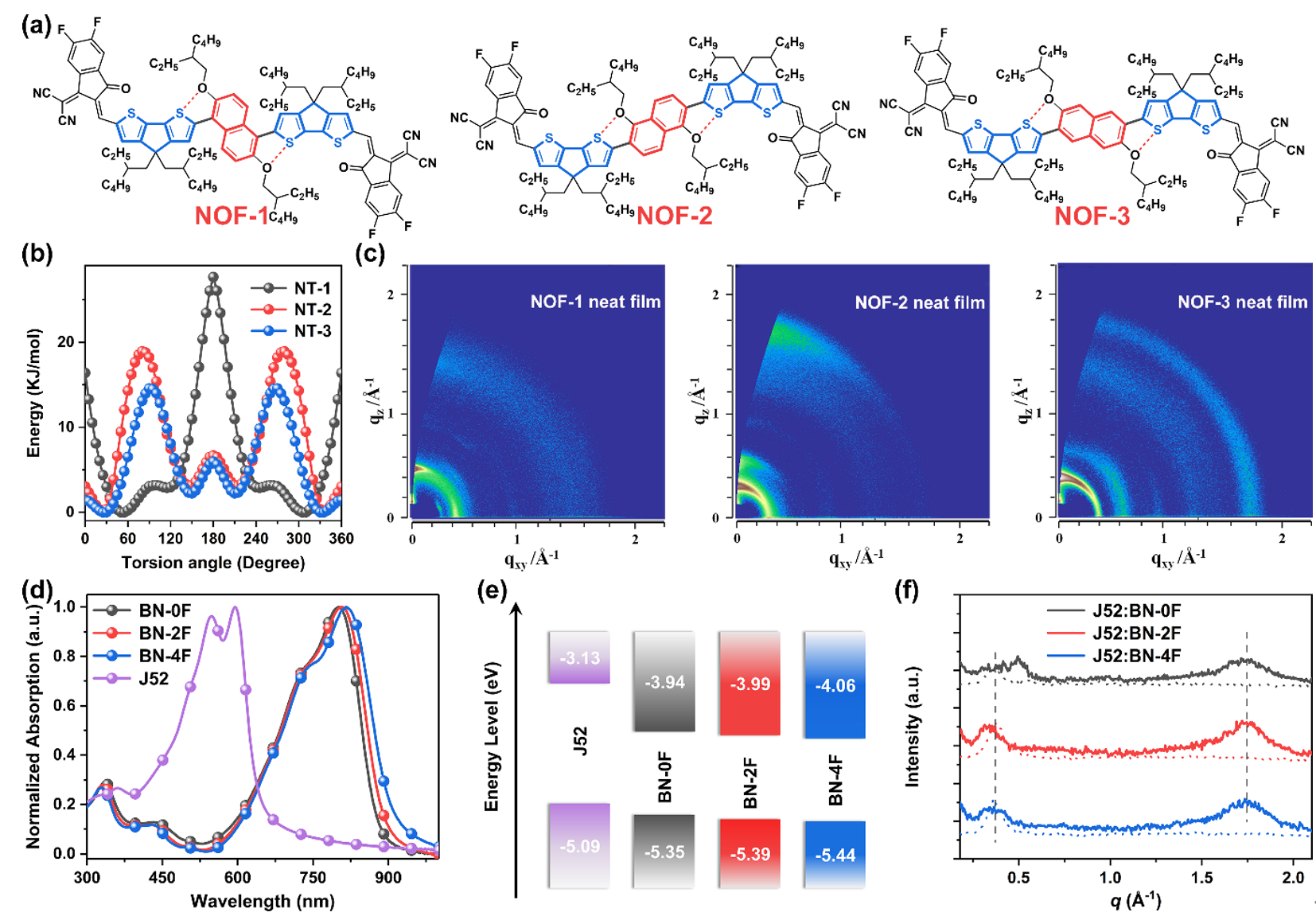
Figure 4. Effects of regioisomerization and end-group engineering on photoelectric properties and stacking behavior
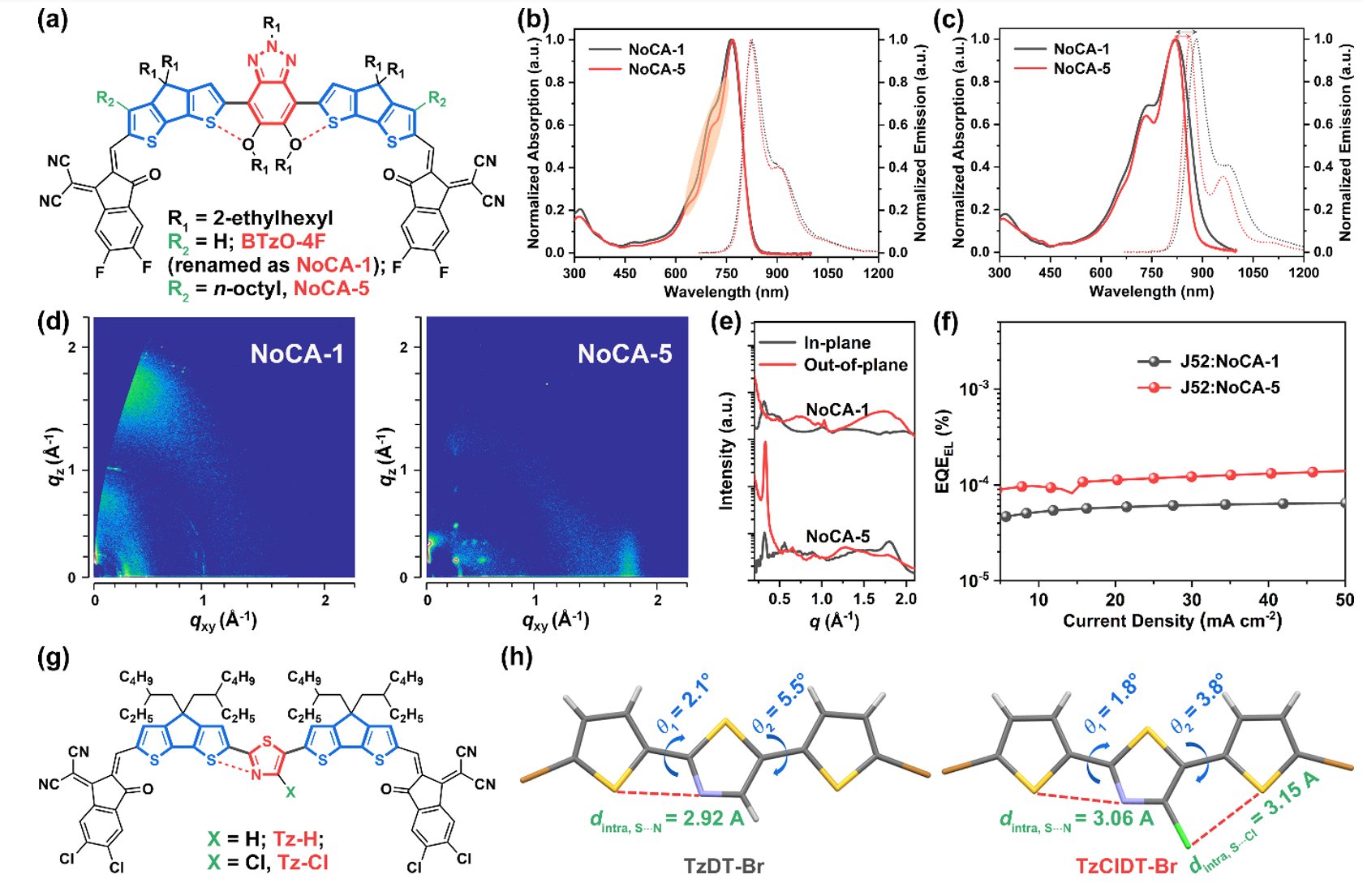
The authors introduce their work on regioisomerization, end group engineering and β-alkyl side chain engineering of non-fused ring electron acceptors, elucidating the impact of the above strategies on molecular geometry, molecular rigidity, optoelectronic properties, stacking behavior and thin films. Shape effects. In addition, the author also introduced non-covalent "conformational locks" of sulfur‧‧‧nitrogen and sulfur‧‧‧chlorine into the design of non-fused ring electron acceptors. The single crystal structure proved the existence of non-covalent "conformational locks" and Its contribution to molecular planarity.
4. Efficiency-stability-cost-balanced polynon-fused ring electron acceptor
All-polymer solar cells have significant advantages such as excellent thermodynamics and light stability, and have great potential in practical applications. In recent years, thanks to the introduction of the concept of polysmall molecule acceptors (usually using fused ring electron acceptors as basic building blocks), the photoelectric conversion efficiency of all-polymer solar cells based on polyfused ring electron acceptors has exceeded 19%. However, this type of receptor materials still faces problems of complex synthesis and high cost. Therefore, considering the advantages of non-fused ring electron acceptors in reducing material costs, the authors first proposed a strategy of polynon-fused ring electron acceptors (PNFREAs), aiming to improve the balance between efficiency, stability and cost (i.e. The “golden triangle” of organic solar cell commercialization).
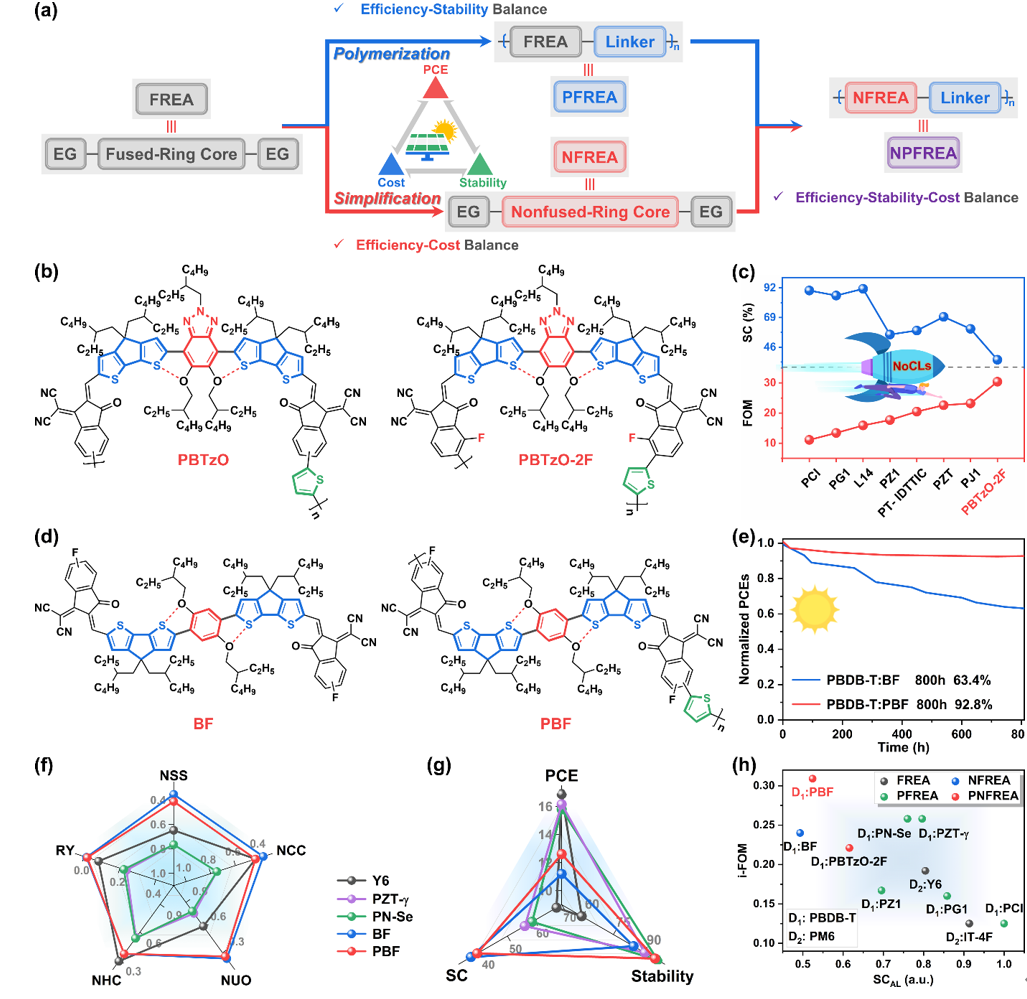
Figure 6. Design idea diagram of polynon-fused ring electron acceptor and its chemical structure, photovoltaic performance, stability and cost analysis
The author summarized the work on polynon-fused ring electron acceptors, analyzed the effects of regional regularity of polymerization sites, terminal fluorination, etc. on its photoelectric properties and photovoltaic performance, and created 12.61% polynon-fused ring Record efficiency for electron acceptor-based all-polymer solar cells. In addition, the author compared the differences in thermal stability and storage stability between small molecule non-fused ring electron acceptors and polynon-fused ring electron acceptors, and conducted an in-depth analysis of the industrial figure of merit (i-FOM value) of relevant acceptor materials. , whose i-FOM value is significantly higher than that of representative systems of fused-ring electron acceptors and their polymeric derivatives). This shows that polynon-fused ring electron acceptors can achieve a good balance between efficiency, stability and cost, and are a promising path towards commercialization of organic solar cells.
【Summary and Outlook】
This review comprehensively reviews their efforts in structural simplification, molecular engineering, and polymerization of low-cost non-fused ring electron acceptors, and introduces the effectiveness of introducing non-covalent “conformational locks” in enhancing molecular planarity and rigidity. properties, which in turn promotes ordered molecular assembly, efficient π conjugation and good charge transport properties. Furthermore, the great potential of polynon-fused ring electron acceptors in achieving efficiency-stability-cost balance in organic solar cells is elucidated. In order to further improve the photovoltaic performance of non-fused ring electron acceptor-based organic solar cells, the author puts forward relevant prospects: 1) further expand the application of new non-covalent "conformational locks" in molecular design based on theoretical guidance; 2) The use of non-covalent "conformational locks" and the double locking effect of large steric hindrance groups helps to develop highly planar non-fused ring electron acceptors; 3) The development of low-cost polymer donors and fully low-cost donors The combination of acceptors is crucial to further reducing the cost of organic solar cells; 4) The use of green and atom-economical synthesis methods helps to shorten the synthesis route, avoid the generation of toxic by-products, and further reduce the synthesis complexity factor; 5) Develop more Photovoltaic receptor materials with commercial potential.
Link:https://pubs.acs.org/doi/10.1021/acs.accounts.3c00813


