The charge transport process has a great impact and even determines the performance of organic solar cells, so building efficient charge transport channels is crucial to realizing high-performance organic photovoltaic devices. In recent years, the invention of fused-ring electron acceptors (represented by ITIC and Y6) has driven the rapid development of the field of organic solar cells, which is inseparable from their ordered/three-dimensional aggregated structure. Compared with fused-ring electron acceptors, non-fused-ring electron acceptors have significant advantages such as simpler chemical structures and convenient synthetic routes, and therefore show great potential for application in low-cost organic solar cells. However, the current photoelectric conversion efficiency of organic photovoltaic devices based on this type of acceptor material still lags behind that of fused-ring electron acceptors. In particular, there is a lack of effective methods to regulate the aggregated structure of non-fused-ring electron acceptors.
Recently, the organic photovoltaic research group of the research group adopted a variety of molecular design strategies to systematically regulate the aggregated structure of non-condensed ring electron acceptors, successfully constructed tightly ordered molecular self-assemblies and highly interconnected charge transfer channels, and broke the efficiency record of organic solar devices based on low-cost non-condensed ring electron acceptors several times.
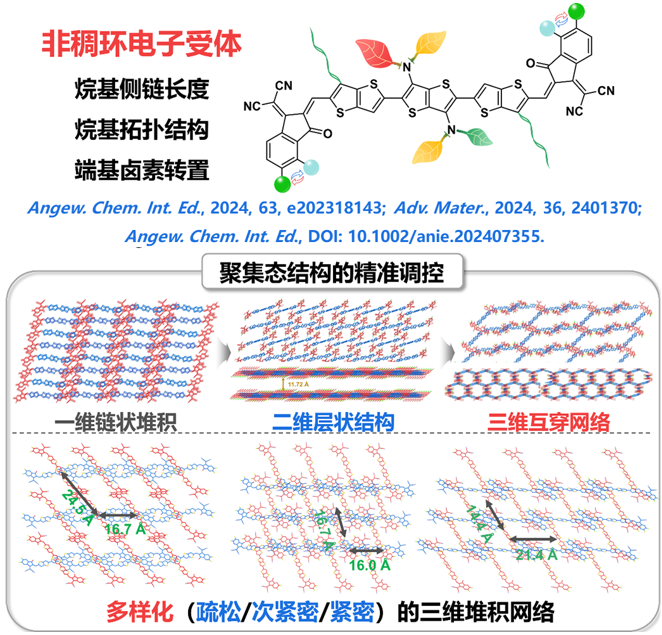
1. Angew. Chem .: Non -common price interactions between multiple molecules helped build a tight three -dimensional gathering collective
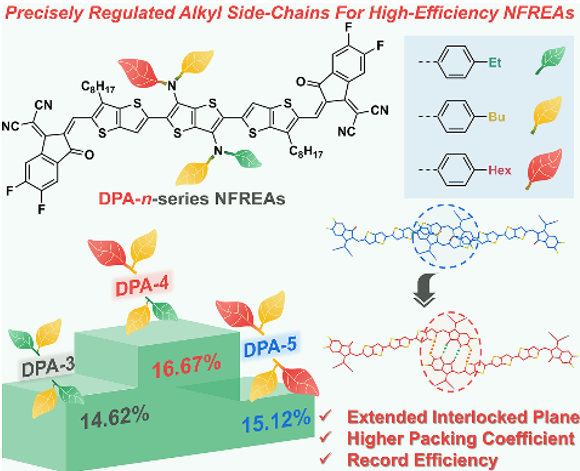
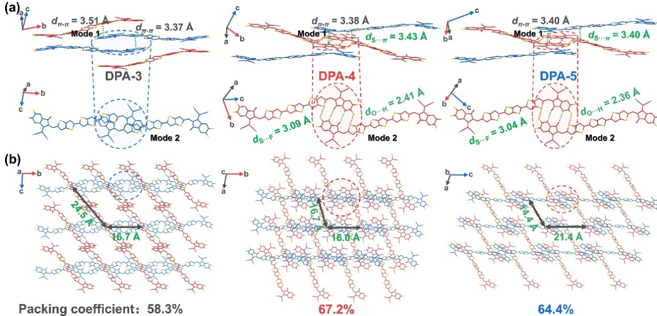
Three new non-thick ring electronic receptors DPA-N (n = 3, 4, 5) were designed and synthesized by finely regulating the length of the dilate-based large position chain. The results of single crystal XRD diffraction analysis show that compared to DPA-3 with the receptor material with the shortest alkyl side chain, the DPA-4 with medium alkyl group has a more tight three-dimensional electronic transmission network and a high accumulation coefficient (67.2%) This is due to the "mutual lock" plane built by multiple molecules non -common price interaction (C = O ··· H and s ·· F). When the alkyl length is further increased, the accumulation coefficient of the DPA-5 shows a decrease (64.4%). In the end, the D18: DPA-4 mixture has more efficient charge transmission, lower energy loss and appropriate phase separation. One of the highest values.
Link:Han, Z.#; Zhang, C.#; He, T.#; Gao, J.; Hou, Y.; Gu, X.; Lv, J.; Yu, N.; Qiao, J.; Wang, S.; Li, C.; Zhang, J.; Wei, Z.; Peng, Q.; Tang, Z.; Hao, X.; Long, G.; Cai, Y.; Zhang, X.*; Huang, H.*, Precisely Manipulating Molecular Packing via Tuning Alkyl Side-Chain Topology Enabling High-Performance Nonfused-Ring Electron Acceptors. Angew. Chem. Int. Ed., 2024, 63, e202318143.
https://doi.org/10.1002/anie.202318143
2. Adv. Mater.: Adjust the alkyl topology structure to build a highly "interconnection" charge transmission channel
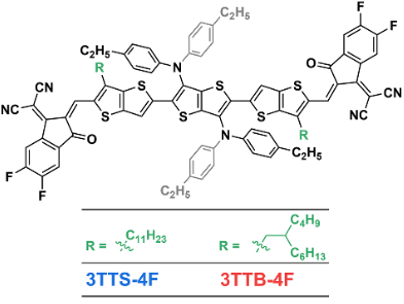
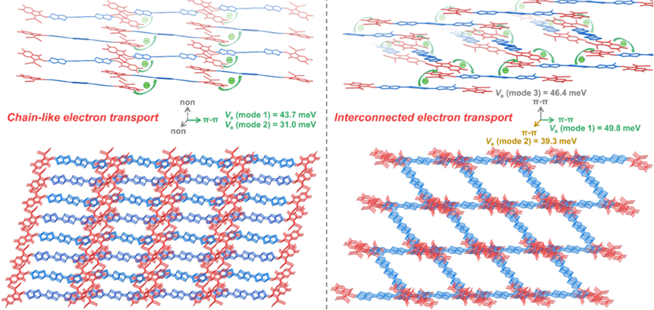
Furthermore, by regulating the alkyl topology of the non-condensed ring electron acceptor, two new non-condensed ring electron acceptors 3TTS-4F and 3TTB-4F were developed. Both have the same conjugated main chain, but the side chains are substituted with straight-chain and branched alkyl groups, respectively. Single crystal XRD diffraction analysis results show that the 3TTS-4F crystal with straight-chain alkyl substitution is π-π stacked in one dimension to form a chain-like charge transfer channel. In contrast, the 3TTB-4F with branched alkyl substitution observes a unique axial rotation π-π stacking, thus forming a highly interconnected charge transfer channel in three dimensions. The results show that the D18:3TTB-4F system has more efficient exciton dissociation and more balanced charge transport, and finally obtains a photoelectric conversion efficiency of up to 17.38% (NPVM verified efficiency is 16.59%), which refreshes the record value of organic photovoltaic devices based on non-condensed ring electron acceptors when reported.
Link:Gu, X.#; Zeng, R.#; He, T.; Zhou, G.; Li, C.; Yu, N.; Han, F.; Hou, Y.; Lv, J.; Zhang, M.; Zhang, J.; Wei, Z.; Tang, Z.; Zhu, H.; Cai, Y.; Long, G.; Liu, F.*; Zhang, X.*; Huang, H.*, Simple-Structured Acceptor with Highly Interconnected Electron-Transport Pathway Enables High-Efficiency Organic Solar Cells. Adv. Mater., 2024, 36, 2401370.
https://doi.org/10.1002/adma.202401370
3. Angew. Chem.: Handalogenic conversion strategies help precise regulation of non -thick ring electron receptor self -assembly structure
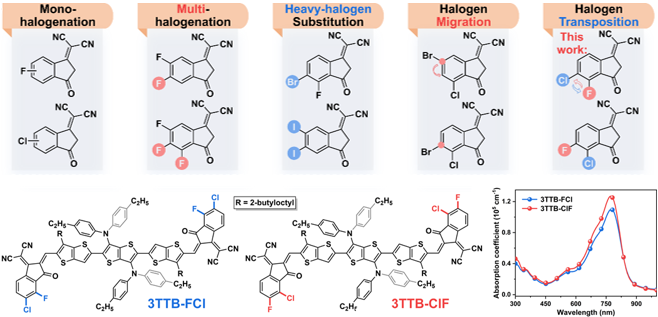
Recently, the team designed and synthesized two new non-thick ring electronic receptors (TTB-FCL and 3TTB-CLF) through halogen reinforcement strategies. UV-visible absorption spectrum display 3TTB-CLF's light absorption coefficient (1.27 × 105 cm−1) in solid state is significantly higher than 3TTB-FCL (1.10 × 105 cm−1), indicating that there is a stronger interaction between its molecules and more Tightly stacked.
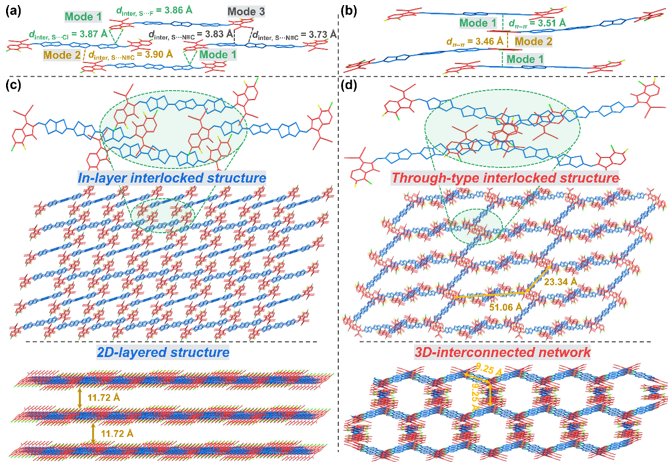
The results of single crystal XRD diffraction analysis show that 3TTB-FCl has an intralayer interlocking structure mediated by multiple intramolecular non-covalent interactions, thus forming a two-dimensional layered structure. In contrast, 3TTB-ClF forms a through-interlocking structure by intermolecular π-π interactions, thus forming a three-dimensional interpenetrating stacking network and building a high-speed channel for charge transfer. Studies have shown that the D18:3TTB-ClF system has higher open circuit voltage, lower energy loss, more efficient exciton dissociation, more balanced charge transport and more suitable phase separation morphology, thus obtaining a photoelectric conversion efficiency of 17.46%. It was further used as the third component to construct a D18:3TTB-4F:3TTB-ClF ternary device, achieving a record efficiency of 18.24%.
Link:Gu, X.#; Zeng, R.#; Hou, Y.; Yu, N.; Qiao, J.; Li, H.; Wei, Y.; He, T.; Zhu, J.; Deng, J.; Tan, S.; Zhang, C.; Cai, Y.; Long, G.; Hao, X.; Tang, Z.; Liu, F.*; Zhang, X.*; Huang, H.*, Precisely Regulating Intermolecular Interactions and Molecular Packing of Nonfused-Ring Electron Acceptors via Halogen Transposition for High-Performance Organic Solar Cells. Angew. Chem. Int. Ed., 2024, e202407355.
https://doi.org/10.1002/anie.202407355.
Summary: The team members of the project group have built a molecular agglomeration that includes one -dimensional, two -dimensional, loose/tight three -dimensional three -dimensional three -dimensional, and several times. The record efficiency has achieved new breakthroughs greater than 18%efficiency for the first time. This series proves the huge application potential of non -thick ring electron receptors and injects new vitality into the development of low -cost organic solar cells.
The above work has been funded by the National Natural Science Foundation of China and the Chinese Academy of Sciences. The author thanks to the strong support for the team of Professor Liu Feng of Shanghai Jiaotong University!


