1 Introduction
In recent years, fused-ring electron acceptors (FREAs) have played a key role in enhancing the device performance of organic solar cells (OSCs), bringing OSCs to a new stage. However, FREAs usually require multiple synthesis steps, resulting in low overall yields and high synthesis costs, which are not conducive to future large-scale commercial applications. In order to simplify the synthetic route and reduce the cost of materials, non-fused ring electron acceptors (NREAs) have emerged. According to the complexity of the π-conjugated nucleus, NREAs can be divided into two types: one type is a simple fused ring as a π-conjugated nucleus. Conjugated nuclei (NREAs-I), another type is non-fused ring π-conjugated nuclei (NREAs-II). However, the performance of NREAs-II is still much lower than that of NREAs-I and FREAs. Therefore, there is an urgent need to explore high-performance NREAs-II through molecular engineering.
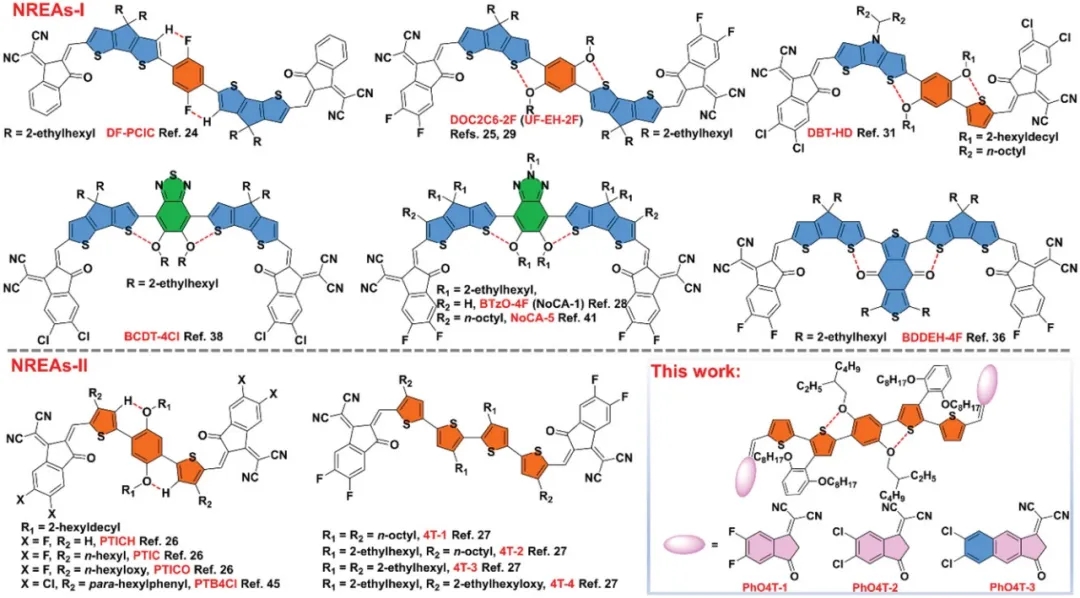
Figure 1: Molecular structure and design strategy
2 Introduction
Based on this, recently, the research team of Professor Huang Hui from the University of Chinese Academy of Sciences designed and synthesized a series of new NREAs-II with S··O covalent bonds: PhO4T-1, PhO4T-2 and PhO4T-3. Using inexpensive and readily available 2,3-dibromothiophene as the starting material, the researchers systematically tuned the light-harvesting ability, energy levels, and stacking behavior of the molecule through different end-group modifications. In thin films, the red shifts of PhO4T-1, PhO4T-2, and PhO4T-3 are about 40, 60, and 50 nm, respectively, indicating that the three molecules can form π-π stacking in the solid state. Meanwhile, PhO4T-3 has the smallest Stokes shift, indicating that PhO4T-3 has the strongest skeleton and the lowest recombination energy. According to the Marcus electron transfer theory, this feature may contribute to electron transport.
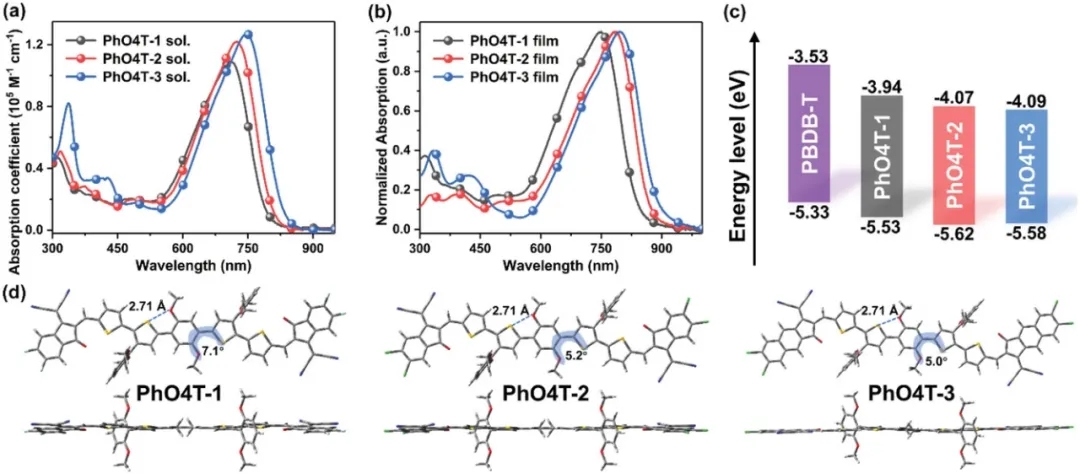
Figure 2. Basic molecular properties
Finally, the researchers prepared the corresponding OSCs devices by blending the three acceptor molecules with the polymer donor PBDB-T, respectively. The results show that the PhO4T-3-based device achieves the best PCE as high as 13.76%, which is mainly attributed to its wide absorption range, near-zero driving force, low-energy disorder, equilibrium mobility, improved phase separation, and good co-location. mixed film form. In addition, the FOM value of PhO4T-3 is much higher than that of high-performance FREAs such as IT-4F, Y6 and M34 through correlation calculation. These results also further illustrate the potential of the developed novel receptor molecules for the preparation of low-cost and high-performance OSCs.
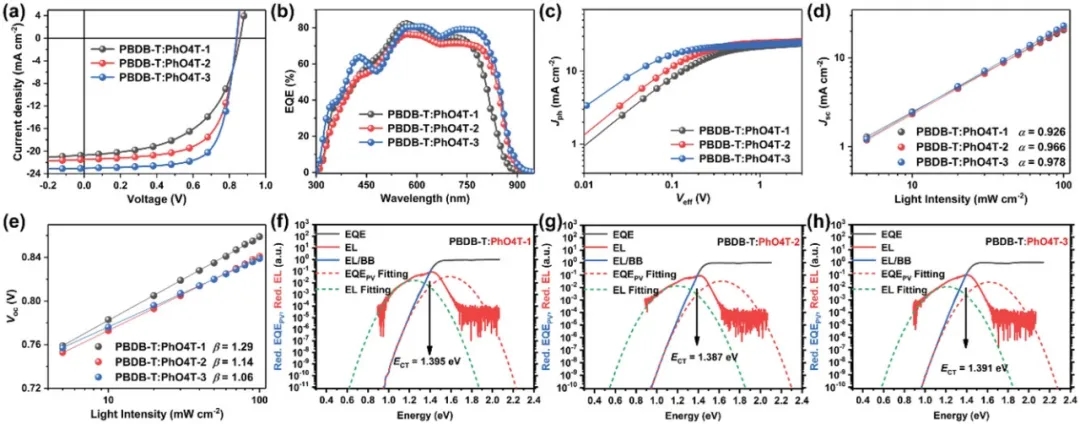
Figure 3. Photovoltaic performance comparison
3 Summary
Taken together, this work presents an important strategy for exploring novel NREAs-II with simple structures. Relevant research results have been published in Advanced Functional Materials, a well-known international academic journal in the field of materials and devices, entitled "Simple Nonfused-Ring Electron Acceptors with Noncovalently Conformational Locks for Low-Cost and High-Performance Organic Solar Cells Enabled by End- Group Engineering".
Keywords in this paper: organic solar cells, non-fused ring electron acceptors, PhO4T-1, PhO4T-2, PhO4T-3.
4 Materials
|
|
PBDB-T | Y6 |